[ad_1]
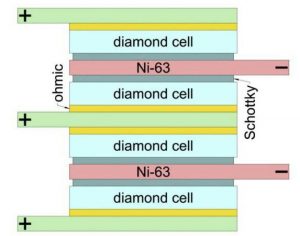
The cell used the beta (fast electron) decay of nickel-63 and diamond Schottkys for energy conversion. The prototype has a stack of 200 diamond converters interleaved with nickel-63 and stable nickel foil layers, and achieved ~1μW output, equating to ~10μW/cm3 – enough for a modern pacemaker, according to the team – which was assembled from the Moscow Institute of Physics and Technology (MIPT), the Technological Institute for Superhard and Novel Carbon Materials (TISNCM), and the National University of Science and Technology MISIS.
Nickel-63 has a half-life of 100 years, which is where the ~3,300mWh/g figure comes from.
“The amount of power generated by the converter depends on the thickness of the nickel foil and the converter itself, because both affect how many beta particles are absorbed. Currently available prototypes of nuclear batteries are poorly optimised, since they have excessive volume,” said MIPT. “If the beta radiation source is too thick, the electrons it emits cannot escape it. This effect is known as self-absorption. However, as the source is made thinner, the number of atoms undergoing beta decay per unit time is proportionally reduced. Similar reasoning applies to the thickness of the converter.”
To maximise power density, the passage of electrons through the beta source and the converters was numerically simulated, revealing that the most effective nickel-63 source is 2μm thick, and the optimal thickness of the Schottky barrier diamond diode converter is ~10μm.
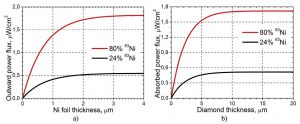 Power flux and structural foil. The graphs indicate that the optimal thicknesses of nickel-63 foil and the diamond converter.
Power flux and structural foil. The graphs indicate that the optimal thicknesses of nickel-63 foil and the diamond converter.
The open-circuit voltage and the short-circuit current are 1.02V and 1.27μA respectively. Maximum output is 0.93μW at 0.92V.
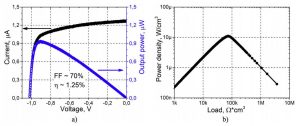 Dependence of current (black) and power (blue) on voltage, and power density as a function of load resistance.
Dependence of current (black) and power (blue) on voltage, and power density as a function of load resistance.
The main fabrication challenge was the large number of diamond conversion cells with complex internal structure.
Conventional mechanical and ionic techniques of diamond thinning were not suitable for this task, so researchers from TISNCM and MIPT developed a way of synthesising thin diamond plates on a diamond substrate and splitting them off to mass-produce ultra-thin converters.
- Boron-doped diamond crystal plates grown as substrates, using a temperature gradient technique under high pressure.
- Ion implantation create a 100nm ‘damaged’ layer in the substrate at depth of ~700nm.
- 15μm boron-doped diamond film grown on top using chemical vapour deposition.
- High-temperature annealing then induces graphitisation of buried defective layer, allowing recovery of top diamond layer.
- Electrochemical etching removes damaged layer, allowing addition of ohmic and Schottky contacts.
“As the operations were repeated, the loss of substrate thickness amounted to no more than 1μm per cycle,” said MIPT. “A total of 200 converters were grown on 20 substrates. This technology is important from an economic standpoint, because high-quality diamond substrates are very expensive and therefore mass-production of converters by substrate thinning is not feasible.”
The technology for rolling 2μm thick nickel foil was developed at the Research Institute and Scientific Industrial Association LUCH.
Finally, the battery was sealed with epoxy.
 “Results so far are already quite remarkable and can be applied in medicine and space technology, but we are planning to do more,” said TISNCM director, and MIPT nanostructure physics chair, Vladimir Blank. “In the recent years, our institute has been rather successful in the synthesis of high-quality doped diamonds, particularly those with n-type conductivity. This will allow us to make the transition from Schottky barriers to p-i-n structures and thus achieve three times greater battery power. The higher the power density of the device, the more applications it will have. We have decent capabilities for high-quality diamond synthesis, so we are planning to utilise the unique properties of this material for creating radiation-proof electronic components and designing novel electronic and optical devices.”
“Results so far are already quite remarkable and can be applied in medicine and space technology, but we are planning to do more,” said TISNCM director, and MIPT nanostructure physics chair, Vladimir Blank. “In the recent years, our institute has been rather successful in the synthesis of high-quality doped diamonds, particularly those with n-type conductivity. This will allow us to make the transition from Schottky barriers to p-i-n structures and thus achieve three times greater battery power. The higher the power density of the device, the more applications it will have. We have decent capabilities for high-quality diamond synthesis, so we are planning to utilise the unique properties of this material for creating radiation-proof electronic components and designing novel electronic and optical devices.”
The work has been published in the journal Diamond and Related Materials.
Some history – provided by the research team
In 2016, Russian researchers from MISIS had already presented a prototype betavoltaic battery based on nickel-63. Another working prototype, created at TISNCM and LUCH, was demonstrated at Atomexpo 2017. It had a useful volume of 1.5cm3.
“There is an alternative radioisotope for use in nuclear batteries: Dimond converters could be made using radioactive carbon-14, which has an extremely long half-life of 5,700 years. Work on such generators was earlier reported by physicists from the University of Bristol,” said MIPT.
Applications are foreseen in ‘perpetual pacemakers’ and spacecraft – diamond is one of the most radiation-proof semiconductors and due to its large bandgap, can operate in a wide range of temperatures.
Further research includes enriching the nickel-63 to increase battery power and developing a diamond p-i-n structure with a controlled doping profile which could boost voltage – potentially tripling output power. Enhancing the surface area of the converter would increase the number of nickel-63 atoms on each converter.
In 1913, Henry Moseley invented the first power generator based on radioactive decay.
His nuclear battery consisted of a glass sphere silvered on the inside with a radium emitter mounted at the centre on an isolated electrode.
Electrons resulting from the beta decay of radium caused a potential difference between the silver film and the central electrode. However, the idle voltage of the device was way too high – tens of kV – and the current was too low for practical applications.
In 1953, Paul Rappaport proposed the use of semiconducting materials to convert the energy of beta decay into electricity. Beta particles – electrons and positrons – emitted by a radioactive source ionize atoms of a semiconductor, creating uncompensated charge carriers.
In the presence of a static field of a p-n structure, the charges flow in one direction, resulting in an electric current.
Batteries powered by beta decay came to be known as betavoltaics. Their chief advantage over galvanic cells being longevity: power output remains nearly constant for a very long time.
Power density of betavoltaic cells is significantly lower than galvanic counterparts. Despite this, betavoltaics were used in the ’70s to power cardiac pacemakers, before being phased out by cheaper lithium-ion batteries, even though the latter have shorter lifetimes.
Betavoltaic power sources should not be confused with radioisotope thermoelectric generators, or RTGs, which are also called nuclear batteries but operate on a different principle.
Thermoelectric cells convert the heat released by radioactive decay into electricity using thermocouples – efficiency is only several percent and depends on temperature. Owing to their longevity and relatively simple design, thermoelectric power sources are widely used to power spacecraft such as the New Horizons probe and Mars rover Curiosity. RTGs were used on unmanned remote facilities such as lighthouses and automatic weather stations. However, this practice was abandoned, because used radioactive fuel was hard to recycle and leaked into the environment.
[ad_2]
Source link